Production of Serratiopeptidase by engineered Escherichia coli strain for therapeutic application
Background
Serratiopeptidase is one of the important proteases which belong to the serine protease family. It is an extracellular zinc-containing metalloprotease that is produced by Serratia marcescens having molecular weight of about 53kD. It has shown therapeutic (anti-inflammatory, anti-fibrinolytic and analgesic) as well as industrial applications (detergents, food processing, leather, paper and brewing etc.). The evolution of Serratia marcescens as an opportunistic pathogen associated with various infections has led researchers to think and develop an alternate strategy for its industrial production. Serratiopeptidase is also taken as a supplement to augment the overall health of the cardiovascular system. It has also shown to reduce the cancer metastasis by thinning its extracellular matrix as well as to solubilize non-living tissues such as mucous, plaques and blood clots. It has the potential to cure and treat several disorders such as atherosclerosis, arthritis, bronchitis, carpal tunnel syndrome, fibrocystic breast disease, Crohn’s disease, leg ulcers, traumatic swelling, fibromyalgia, breast engorgement, migraine, Alzheimer’s disease, sinusitis, hepatitis, lung disorders, arthritis, diabetes, carotid artery blockage, thrombosis, uterine fibroids. Currently the industrial production of serratiopeptidase makes use of natural S. marcescens strain. However, many challenges and issues have arisen to use this strain because of pathogenic nature, lack of genetic tools, unclear mechanism of the utilization of cheaper carbon and nitrogen sources, industrial -scale fermentation and process. The bacteria is also reported to cause pneumonia, septicemia, and as well is associatedwith cystic fibrosis. Therefore, a pressing need hasarisen to find an alternative approach for the production of active and efficient serratiopeptidase
Technology
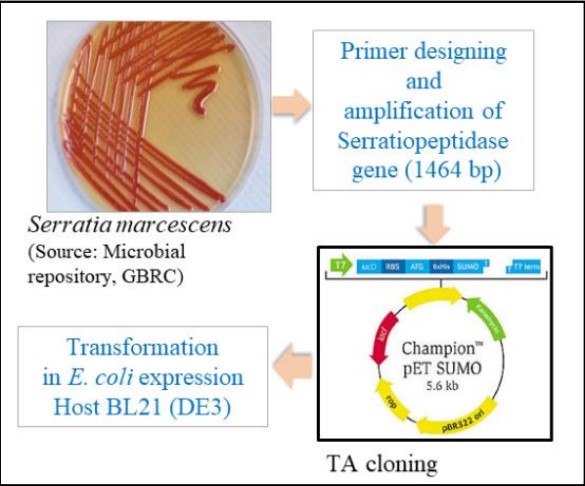
GBRC make successful cloning, expression and purification of active serratiopeptidase, using Escherichia coli BL21 [DE3] and pET SUMO vector. Further it also optimize synthetic media and culture conditions for enhanced serratiopeptidase production. Initial optimization of physical parameters was done followed by a screening of different carbon and nitrogen sources. The significant media components for serratiopeptidase production as shown by factorial screening experiment were subjected to Response Surface Methodology (RSM) based optimization.
Potential Applications
The success of the application of a statistical model for designing an optimized media for enhanced serratiopeptidase production also suggests a new insight for the scale up of serratiopeptidase towards industrial applications.
Technology Status
Technology is ready and the optimized media yielded 2.5 ± 0.764 g L-1 of protein was obtained having 8382±291 U mg-1 of specific caseinolytic activity.