Research Areas
Industrial Biotechnology
Ongoing Projects
Technology Development for Nattokinase production
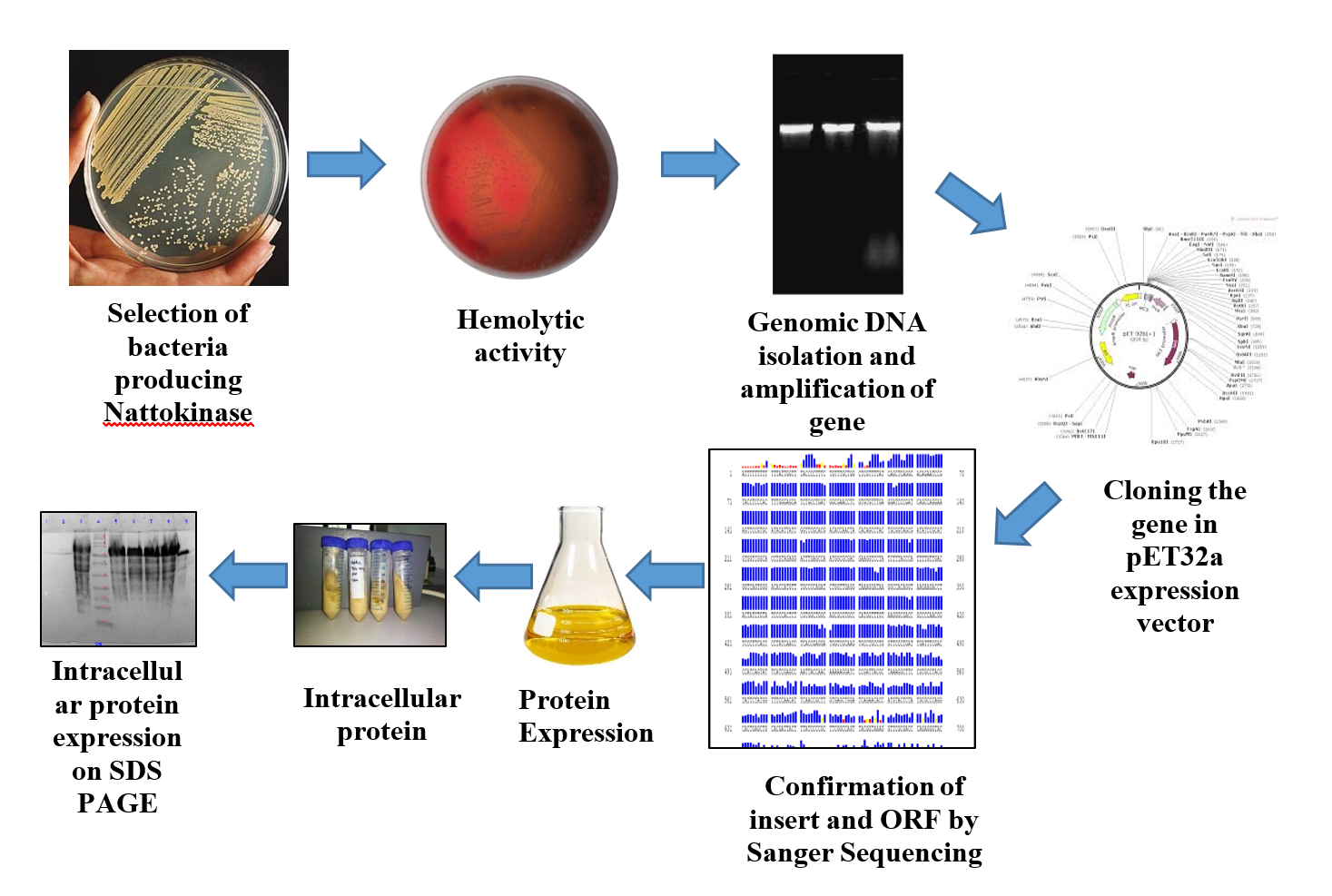 Nattokinase is an enzyme extracted from a popular Japanese food called Natto. Nattokinase (NK) is a serine protease possessing many key beneficial effects on the cardiovascular system. Due to its anti-hypertensive, anti-atherosclerotic and anti-coagulant activity, it has been widely used as an oral therapeutic for cardiovascular diseases. There are reports of NK being absorbed efficiently via the gastrointestinal tract. A strong fibrinolytic activity was induced after its oral administration. Our main objective of this study is development of technology for the overproduction of nattokinase using recombinant DNA technology, for that purpose cloning of nattokinase gene from Bacillus subtilis was done in expression vector and expression host E. coli BL21 (DE3). Protein expression was done using IPTG as inducer and media optimization done for the overproduction of recombinant nattokinase using Central Composite Design - Response Surface Methodology. Protein purification was done using Ni-NTA affinity chromatography and biologically active protein was obtained which was further analysed using SDS PAGE gel. As nattokinase has therauptic applications so for that purpose fibrinolytic and hemolytic activity was done to evaluate the potential application of nattokinase.
Nattokinase is an enzyme extracted from a popular Japanese food called Natto. Nattokinase (NK) is a serine protease possessing many key beneficial effects on the cardiovascular system. Due to its anti-hypertensive, anti-atherosclerotic and anti-coagulant activity, it has been widely used as an oral therapeutic for cardiovascular diseases. There are reports of NK being absorbed efficiently via the gastrointestinal tract. A strong fibrinolytic activity was induced after its oral administration. Our main objective of this study is development of technology for the overproduction of nattokinase using recombinant DNA technology, for that purpose cloning of nattokinase gene from Bacillus subtilis was done in expression vector and expression host E. coli BL21 (DE3). Protein expression was done using IPTG as inducer and media optimization done for the overproduction of recombinant nattokinase using Central Composite Design - Response Surface Methodology. Protein purification was done using Ni-NTA affinity chromatography and biologically active protein was obtained which was further analysed using SDS PAGE gel. As nattokinase has therauptic applications so for that purpose fibrinolytic and hemolytic activity was done to evaluate the potential application of nattokinase.
MetaXtreme: Discovery of 10 (ten) hyper thermostable enzymes
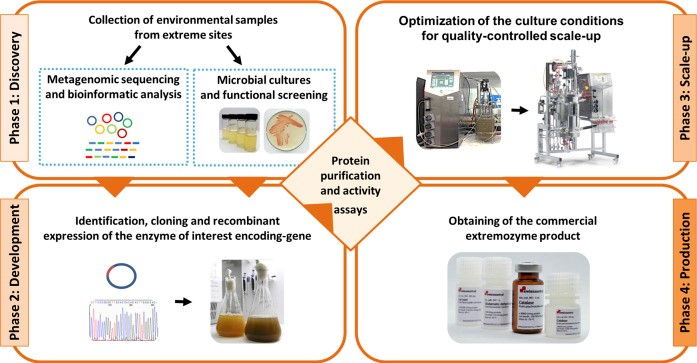 Extremophilic microorganisms present the novel and underexplored source for the important enzymes. The extremophiles living in extreme environments have developed unique molecular mechanisms for the survival in extremities of pH, temperature, salinity and radiation. Extremozymes- extremophile derived enzymes- are able to catalyze the reaction under the conditions similar to the industrial processes and offer alternatives for industrial applications as well as represent the cornerstone for the development of environmentally friendly, efficient, and sustainable industrial technologies. Four industrially important enzymes genes – α-amylase, α-glucosidase, β-glucosidase and α-xylosidase were obtained from the metagenomes of the Tuwa and Unnai hot springs. Enzymes were cloned in pET32a vector and expressed in Escherichia coli BL21 (DE3) strain. Protein expression by IPTG induction was observed and optimized at lower temperature. Protein purification was done using Ni-NTA affinity chromatography and biologically active protein was obtained which was further analyzed using SDS PAGE gel. Many more important enzymes having the potential application in food, fermentation, detergent, paper and pharmaceutical industry are to be identified and expressed from the metagenomes of hot springs.
Extremophilic microorganisms present the novel and underexplored source for the important enzymes. The extremophiles living in extreme environments have developed unique molecular mechanisms for the survival in extremities of pH, temperature, salinity and radiation. Extremozymes- extremophile derived enzymes- are able to catalyze the reaction under the conditions similar to the industrial processes and offer alternatives for industrial applications as well as represent the cornerstone for the development of environmentally friendly, efficient, and sustainable industrial technologies. Four industrially important enzymes genes – α-amylase, α-glucosidase, β-glucosidase and α-xylosidase were obtained from the metagenomes of the Tuwa and Unnai hot springs. Enzymes were cloned in pET32a vector and expressed in Escherichia coli BL21 (DE3) strain. Protein expression by IPTG induction was observed and optimized at lower temperature. Protein purification was done using Ni-NTA affinity chromatography and biologically active protein was obtained which was further analyzed using SDS PAGE gel. Many more important enzymes having the potential application in food, fermentation, detergent, paper and pharmaceutical industry are to be identified and expressed from the metagenomes of hot springs.
Scale up production of important biopharmaceuticals: Recombinant tissue plasminogen activator (tPA) and hyaluronidase
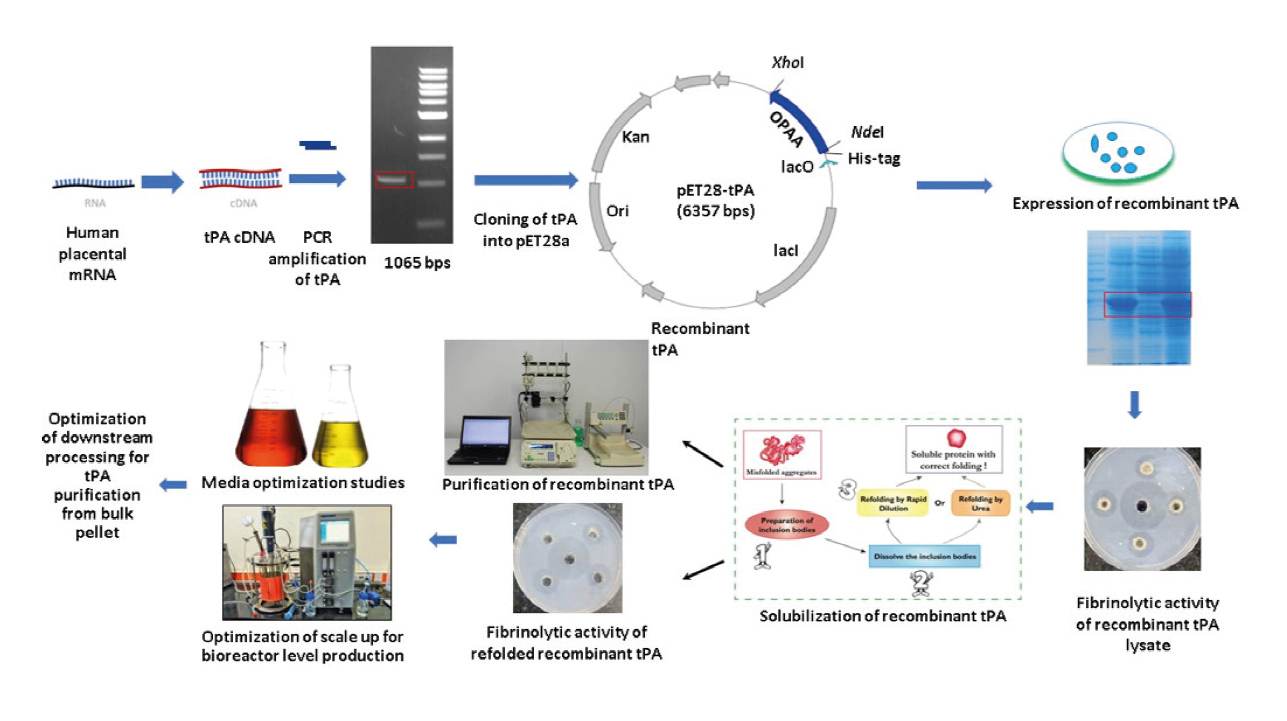 The project focuses on advancing pivotal biopharmaceuticals, including tissue plasminogen activator (tPA) and hyaluronidase enzymes. Initial steps involve cloning tPA and hyaluronidase gene constructs into the pET28a expression vector. Subsequent phases encompass comprehensive exploration of expression, purification, and activity profiles of these enzymes. Media optimization studies are conducted to fine-tune environmental conditions for maximal production efficiency. Scaling up efforts are directed towards optimizing bioreactor-level production of recombinant tPA and hyaluronidase, addressing industrial requirements. Furthermore, downstream processing techniques are refined to ensure efficient purification from bulk pellet, thereby enhancing overall process efficacy. This systematic approach aims to contribute to the advancement of biopharmaceutical production methodologies with potential implications for biomedical applications.
The project focuses on advancing pivotal biopharmaceuticals, including tissue plasminogen activator (tPA) and hyaluronidase enzymes. Initial steps involve cloning tPA and hyaluronidase gene constructs into the pET28a expression vector. Subsequent phases encompass comprehensive exploration of expression, purification, and activity profiles of these enzymes. Media optimization studies are conducted to fine-tune environmental conditions for maximal production efficiency. Scaling up efforts are directed towards optimizing bioreactor-level production of recombinant tPA and hyaluronidase, addressing industrial requirements. Furthermore, downstream processing techniques are refined to ensure efficient purification from bulk pellet, thereby enhancing overall process efficacy. This systematic approach aims to contribute to the advancement of biopharmaceutical production methodologies with potential implications for biomedical applications.
Gujarat Repository of Biomolecules
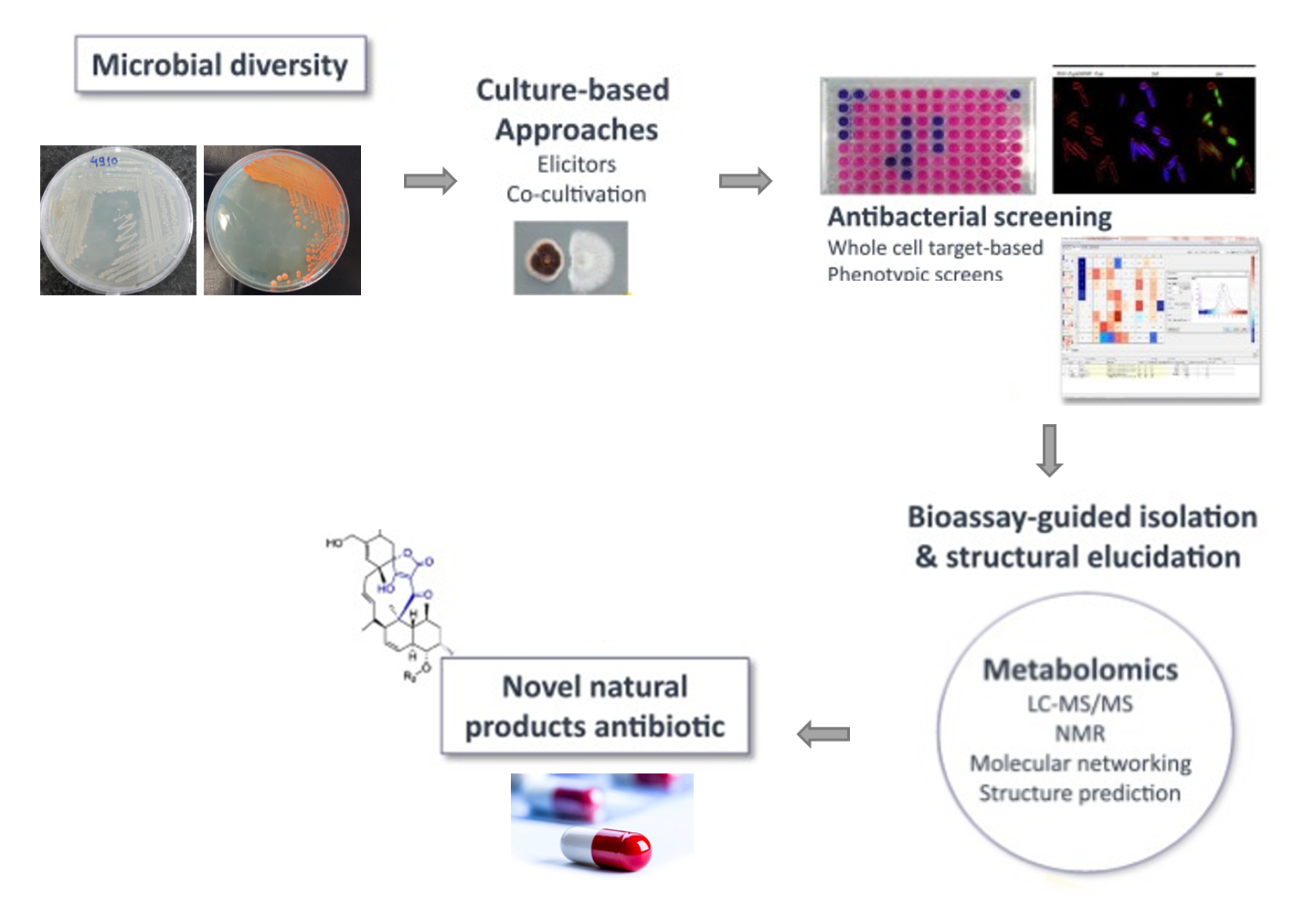 Dermatophytes are a group of primary pathogenic fungi responsible for the common fungal infections in humans. The disease burden of chronic relapsing and therapy refractory superficial dermatophytosis dramatically increased in India during the last decade. Over the past few years, healthcare professionals in India have witnessed a significant increase in the number of patients presenting with dermatophytoses, as well as in the number of difficult-to-treat and recalcitrant cases. The latter has been attributed to multiple causes including an abuse of irrational fixed drug combination creams containing potent steroids, an altered immune response of the host, and microbiological resistance of the causative fungi. 3 dermatophytes were isolated from the clinical specimens and found to be resistant to multiple antifungals used in the treatment. The inhibition of the dermatophytes by Strpetomyces spp. was observed and metabolites were identified using GC-MS. Pigments produced by several isolates also possess antimicrobial activity against specific dermatophytes. Antifungal metabolites and pigment characterization will help to find the alternatives for the AMR.
Dermatophytes are a group of primary pathogenic fungi responsible for the common fungal infections in humans. The disease burden of chronic relapsing and therapy refractory superficial dermatophytosis dramatically increased in India during the last decade. Over the past few years, healthcare professionals in India have witnessed a significant increase in the number of patients presenting with dermatophytoses, as well as in the number of difficult-to-treat and recalcitrant cases. The latter has been attributed to multiple causes including an abuse of irrational fixed drug combination creams containing potent steroids, an altered immune response of the host, and microbiological resistance of the causative fungi. 3 dermatophytes were isolated from the clinical specimens and found to be resistant to multiple antifungals used in the treatment. The inhibition of the dermatophytes by Strpetomyces spp. was observed and metabolites were identified using GC-MS. Pigments produced by several isolates also possess antimicrobial activity against specific dermatophytes. Antifungal metabolites and pigment characterization will help to find the alternatives for the AMR.
Completed Projects
Technology development for Streptokinase production
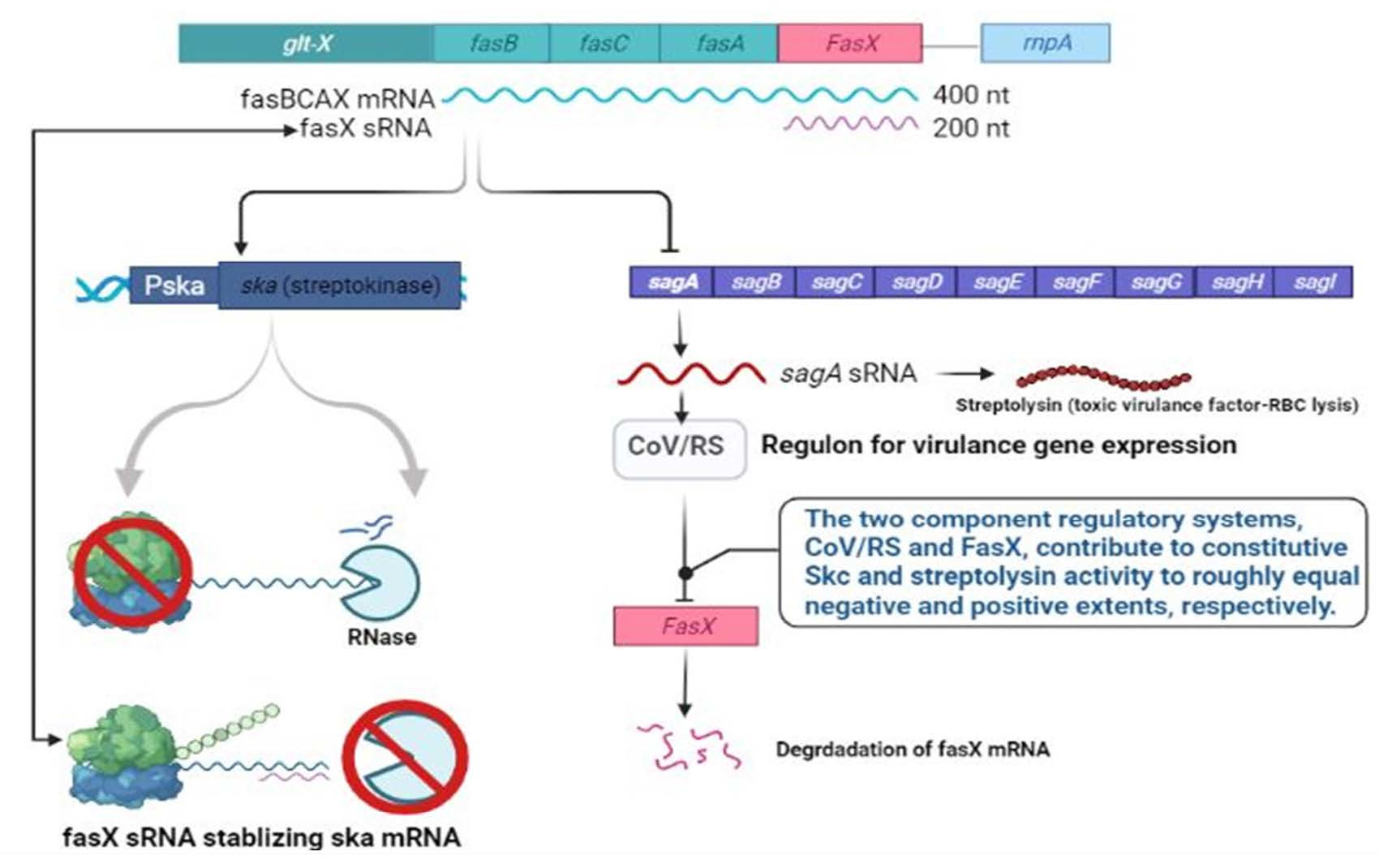 Streptokinase is an enzyme used to break down the blood clots in some cases of myocardial infarction, pulmonary embolism, and arterial thromboembolism. Global Demand for streptokinase is high due to the increased incidences of various heart conditions. Streptococcus strains are the main source of the enzyme streptokinase. Expression of streptokinase in native strain Streptococcus equisimilis is limited due to the SagD gene encoding streptolysin, an inhibitor of streptokinase expression through degradation of FasX small RNA which stabilizes streptokinase mRNA. Using CRISPR-Cas9, we successfully knockout the SagD gene that resulted in 13.58-fold increased expression of streptokinase at the transcript level and 1.48-fold higher expression at the protein level in the mutant strain. Engineered S. equisimilis can be further used for overexpression of streptokinase for therapeutic applications.
Streptokinase is an enzyme used to break down the blood clots in some cases of myocardial infarction, pulmonary embolism, and arterial thromboembolism. Global Demand for streptokinase is high due to the increased incidences of various heart conditions. Streptococcus strains are the main source of the enzyme streptokinase. Expression of streptokinase in native strain Streptococcus equisimilis is limited due to the SagD gene encoding streptolysin, an inhibitor of streptokinase expression through degradation of FasX small RNA which stabilizes streptokinase mRNA. Using CRISPR-Cas9, we successfully knockout the SagD gene that resulted in 13.58-fold increased expression of streptokinase at the transcript level and 1.48-fold higher expression at the protein level in the mutant strain. Engineered S. equisimilis can be further used for overexpression of streptokinase for therapeutic applications.
Technology Development for Serratiopeptidase production
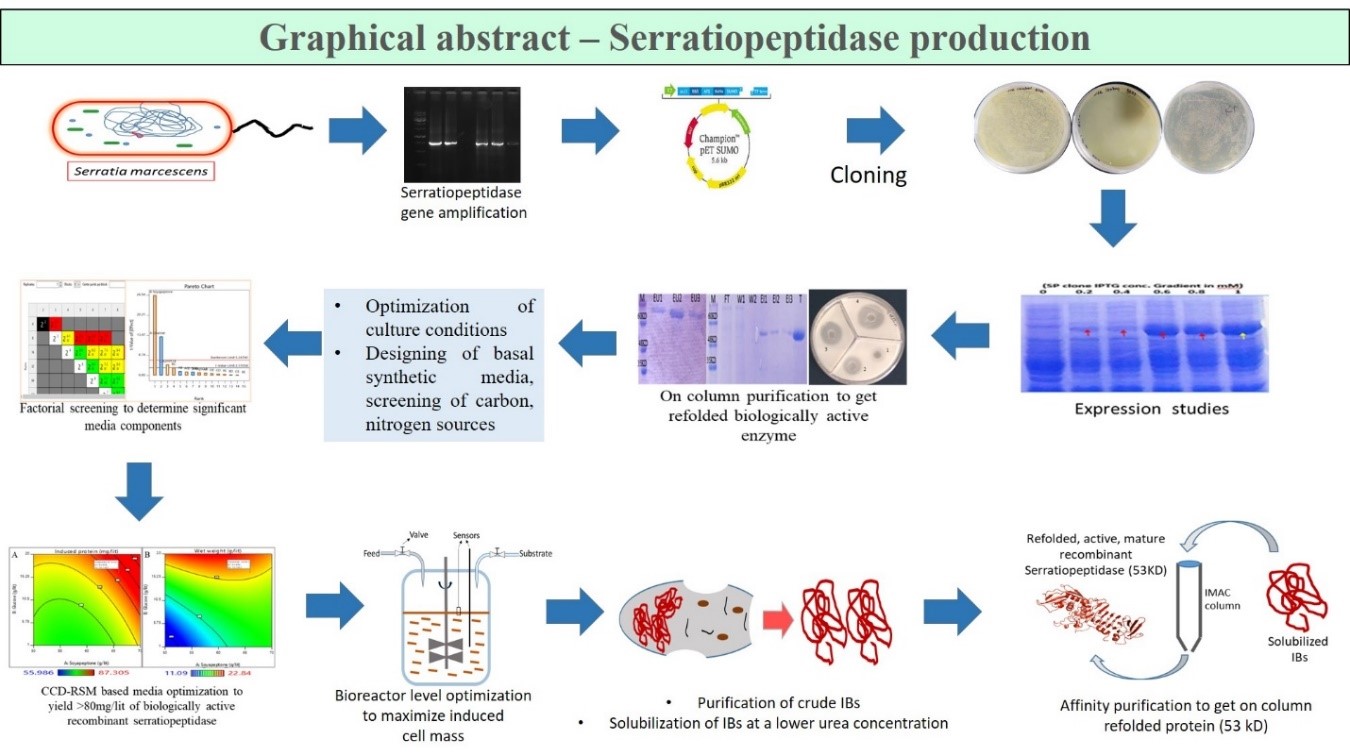 Serratiopeptidase, also known as a miracle enzyme, has already proved its potential as an anti-inflammatory, mucolytic, fibrinolytic, analgesic in many studies. Cloning of serratiopeptidase gene was done in pET SUMO vector followed by the expression and purification studies. On column purification protocol was optimized for refolding the protein from inclusion bodies. The purified enzyme was found to be refolded and showed proteolytic and fibrinolytic activity. Statistical media optimization for the production of the recombinant serratiopeptidase was done by the factorial screening of the significant media components for serratiopeptidase production. Significant components were subjected to Response Surface Methodology (RSM) based optimization. It was followed by a cost effective, bioreactor level production process comprising of the fed-batch fermentation optimization process to produce recombinant serratiopeptidase protein expressed as a fusion construct. High yield of cell mass as well as protein was obtained by the optimization of bioreactor parameters. The downstream solubilization and purification processes were also optimized to achieve maximum yield of pure, active serratiopeptidase protein. A final yield of 2.5 ± 0.764 g L−1 of protein was obtained, having 8382±291 U mg−1 of specific caseinolytic activity. The purified mature serratiopeptidase was further confirmed by MALDI MS/MS analysis. Additionally, a novel, unexpected self-proteolytic activity of the enzyme that cleaves the N-terminal 6× His-SUMO fusion tag along with the enzyme propeptide, thus yielding a mature serratiopeptidase, was also found.
Serratiopeptidase, also known as a miracle enzyme, has already proved its potential as an anti-inflammatory, mucolytic, fibrinolytic, analgesic in many studies. Cloning of serratiopeptidase gene was done in pET SUMO vector followed by the expression and purification studies. On column purification protocol was optimized for refolding the protein from inclusion bodies. The purified enzyme was found to be refolded and showed proteolytic and fibrinolytic activity. Statistical media optimization for the production of the recombinant serratiopeptidase was done by the factorial screening of the significant media components for serratiopeptidase production. Significant components were subjected to Response Surface Methodology (RSM) based optimization. It was followed by a cost effective, bioreactor level production process comprising of the fed-batch fermentation optimization process to produce recombinant serratiopeptidase protein expressed as a fusion construct. High yield of cell mass as well as protein was obtained by the optimization of bioreactor parameters. The downstream solubilization and purification processes were also optimized to achieve maximum yield of pure, active serratiopeptidase protein. A final yield of 2.5 ± 0.764 g L−1 of protein was obtained, having 8382±291 U mg−1 of specific caseinolytic activity. The purified mature serratiopeptidase was further confirmed by MALDI MS/MS analysis. Additionally, a novel, unexpected self-proteolytic activity of the enzyme that cleaves the N-terminal 6× His-SUMO fusion tag along with the enzyme propeptide, thus yielding a mature serratiopeptidase, was also found.


